 Loading... Please wait...
Loading... Please wait...Categories
- Home
- Micro Molecules
- D-Glucopyranose Micro Molecule
Chemkits
D-Glucopyranose Micro Molecule
Product Description
With six carbon atoms, it is classed as a hexose, a subcategory of the monosaccharides. d-Glucose is one of the sixteen aldohexose stereoisomers. The d-isomer, d-glucose, also known as dextrose, occurs widely in nature, but the l-isomer, l-glucose, does not. Glucose can be obtained by hydrolysis of carbohydrates such as milk sugar (lactose), cane sugar (sucrose), maltose, cellulose, glycogen, etc. Dextrose is commonly commercially manufactured from cornstarch in the US and Japan, from potato and wheat starch in Europe, and from tapioca starch in tropical areas.[24] The manufacturing process uses hydrolysis via pressurized steaming at controlled pH in a jet followed by further enzymatic depolymerization.[25]Unbonded glucose is one of the main ingredients of honey.
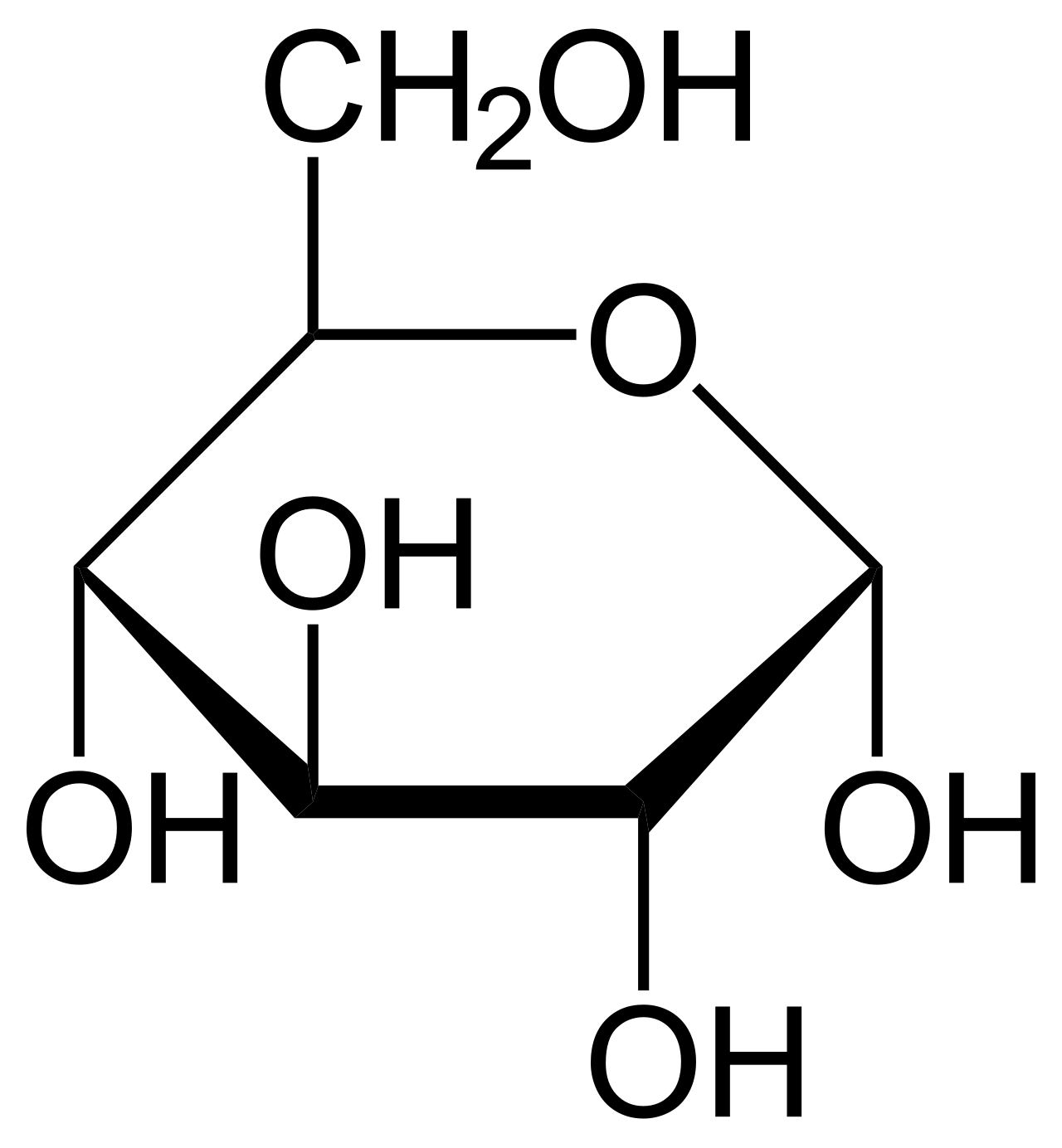
Glucose is usually present in solid form as a monohydrate with a closed pyran ring (dextrose hydrate). In aqueous solution, on the other hand, it is an open-chain to a small extent and is present predominantly as α- or β-pyranose, which interconvert. From aqueous solutions, the three known forms can be crystallized: α-glucopyranose, β-glucopyranose and β-glucopyranose hydrate.[26] Glucose is a building block of the disaccharides lactose and sucrose (cane or beet sugar), of oligosaccharides such as raffinose and of polysaccharides such as starch, amylopectin, glycogen, and cellulose.
CAUTION: THESE MOLECULES CONTAIN MANY SMALL PARTS NOT SUITABLE FOR CHILDREN UNDER 5 YEARS OF AGE.





